+6价Ru氧化物催化水氧化机制研究
(Hexavalent Ru Catalyst with Both Lattice Oxygen and Metal Ion Mechanisms Coactive for Water Oxidation)
Y. Z. Li, J. F. Zhao, S. Zhang, Y. l. Fan, C.-Y. Kuo, Y.-C. Ku, T.-S. Chan, C.-W. Kao, Y.-C. Huang, C.-T. Chen, S.-C. Haw, C. Q. Jin, H. B. Zhao, D. X. Ye, C. Jing, Z. W. Hu, and L. J. Zhang
J. Am. Chem. Soc. 147, 26854 (2025)
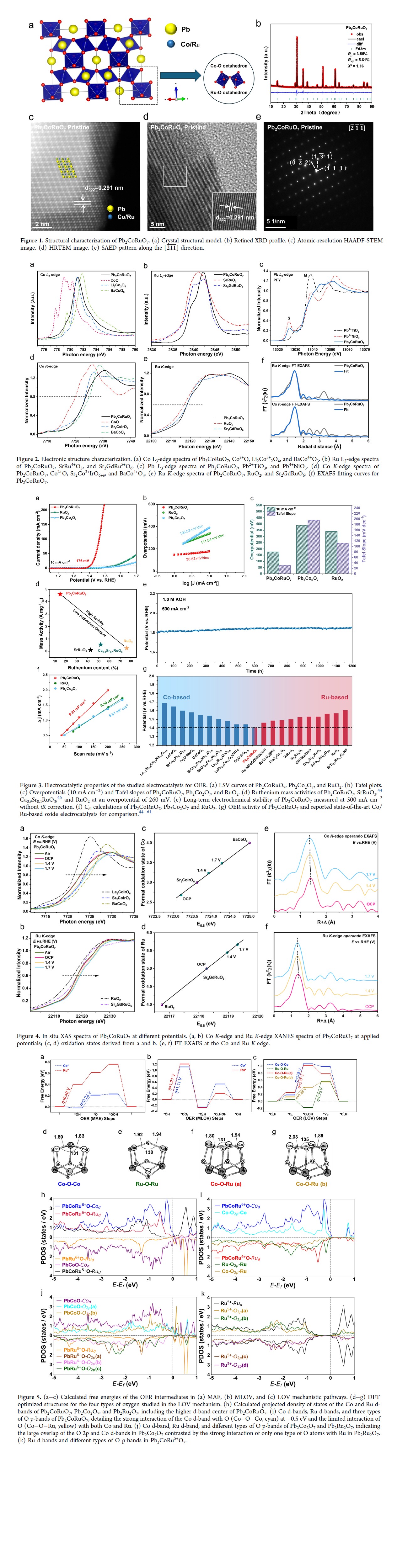
Green hydrogen from water requires the development of efficient and low-cost catalysts for anodic oxygen evolution reaction (OER), which is the main obstacle for electrochemical water splitting. Herein, we focus on an OER catalyst (Pb2CoRuO7) featuring Ru6+, which exhibits an ultralow overpotential of 176 mV at 10 mA cm?2 and a Tafel slope of 30.52 mV dec?1 vs 340 mV at 10 mA cm?2 and a Tafel slope of 111.54 mV dec?1 for RuO2 in 1.0 M KOH solution. In situ X-ray absorption experiments demonstrated the gradual conversion of Ru5+ ions into high-valence Ru6+, while a portion of Co3+ ions transformed into Co4+ during the OER process. Density functional theory calculations revealed that the ultrahigh OER activity of Pb2CoRuO7 was contributed by both metal-site adsorbate evolution (MAE) at the Co site and the latticeoxygen-vacancy-site (LOV) mechanism involving lattice oxygen located between Ru6+ and Co. Our work presents a new and unusual OER catalyst where both the MAE and LOV mechanisms cooperatively facilitate catalytic activity.