新型水合物研制取得重要进展
Research highlights: 中国科学院、中科院物理所
II型冰结构的氖气水合物形成动力学研究
(Crystal structure and encapsulation dynamics of ice II-structured neon hydrate)
X.H. Yu, J.L. Zhu, S.Y. Du, , H.W. Xu, S.C. Vogel, J.T. Han, T.C. Germann, J.Z. Zhang, C.Q. Jin, J.S. Franciscoh, Y.S. Zhao
Proc Natl Acad Sci USA(PNAS) 111, 10456 (2014)
J.L. Zhu, S.Y. Du, X.H. Yu, J.Z. Zhang, H.W. Xu, S.C. Vogel, T.C. Germann, J.S. Francisco, F. Izumi, K. Momma, Y. Kawamura,
C.Q. Jin and Y.S. Zhao
Nature Communications | 5:4128 | (2014)
Crystal structure and encapsulation dynamics of ice II-structured neon hydrate
Neon hydrate was synthesized and studied by in situ neutron diffraction at 480 MPa and temperatures ranging from 260 to 70 K. For the first time to our knowledge, we demonstrate that neon atoms can be enclathrated in water molecules to form ice II-structured hydrates. The guest Ne atoms occupy the centers of D2O channels and have substantial freedom of movement owing to the lack of direct bonding between guest molecules and host lattices. Molecular dynamics simulation confirms that the resolved structure where Ne dissolved in ice II is thermodynamically stable at 480 MPa and 260 K. The density distributions indicate that the vibration of Ne atoms is mainly in planes perpendicular to D2O channels, whereas their distributions along the channels are further constrained by interactions between adjacent Ne atoms.

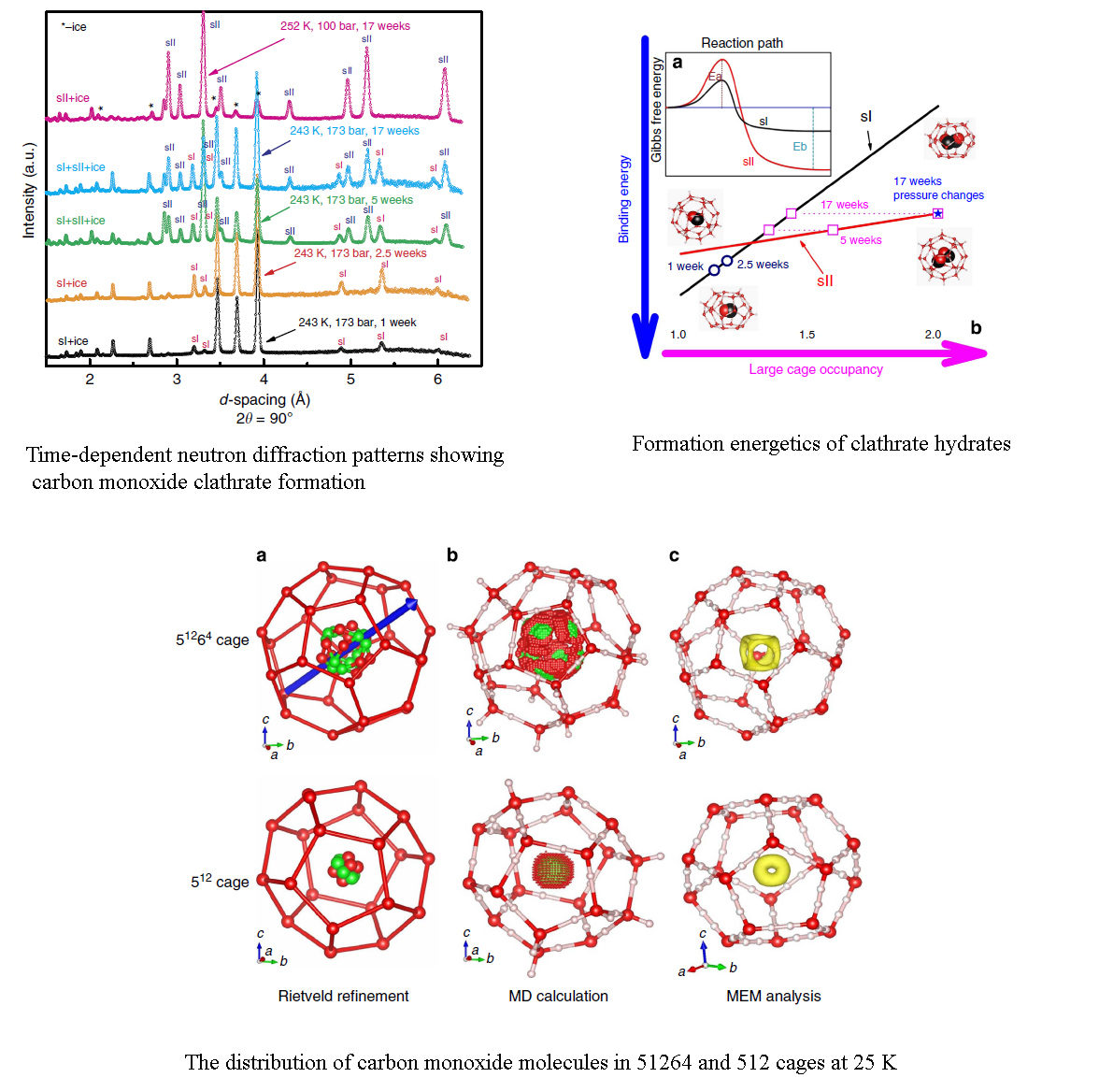
Encapsulation kinetics and dynamics of carbon monoxide in clathrate hydrate
Carbon monoxide clathrate hydrate is a potentially important constituent in the solar system. In contrast to the well-established relation between the size of gaseous molecule and hydrate structure, previous work showed that carbon monoxide molecules preferentially form structure-I rather than structure-II gas hydrate. Resolving this discrepancy is fundamentally important to understanding clathrate formation, structure stabilization and the role the dipole moment molecular polarizability plays in these processes. Here we report the synthesis of structure-II carbon monoxide hydrate under moderate high-pressure/low-temperature conditions. We demonstrate that the relative stability between structure-I and structure-II hydrates is primarily determined by kinetically controlled cage filling and associated binding energies. Within hexakaidecahedral cage, molecular dynamic simulations of density distributions reveal eight low-energy wells forming a cubic geometry in favour of the occupancy of carbon monoxide molecules, suggesting that the carbon monoxide–water and carbon monoxide–carbon monoxide interactions with adjacent cages provide a significant source of stability for the structure-II clathrate framework.